Sodium

Sodium is a chemical element with the symbol Na (from Latin natrium) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite, i.e., rock salt (NaCl). Many salts of sodium are highly water-soluble: sodium ions have accumulated from the leaching action of water on Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans.
Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals including humans.
Quotes
[edit]- Sir Humphrey Davy
Abominated gravy.
He lived in the odium
Of having discovered sodium.- Edmund Clerihew Bentley, Clerihews: Biography for Beginners (1905)

- The first difficulty that faced us was the identification of the forms seen on focusing the sight on gases. We could only proceed tentatively. Thus, a very common form in the air had a sort of dumb-bell shape... we examined this, comparing our rough sketches, and counted its atoms; these, divided by 18—the number of ultimate atoms in hydrogen—gave us 23.22 as atomic weight, and this offered the presumption that it was sodium. We then took various substances—common salt, etc.—in which we knew sodium was present, and found the dumb-bell form in all. In other cases, we took small fragments of metals, as iron, tin, zinc, silver, gold; in others, again, pieces of ore, mineral waters, etc... In all, 57 chemical elements were examined, out of the 78 recognized by modern chemistry.
In addition to these, we found 3 chemical waifs: an unrecognized stranger between hydrogen and helium which we named occultum, for purposes of reference, and 2 varieties of one element, which we named kalon and meta-kalon, between xenon and osmium... Thus we have tabulated in all 65 chemical elements, or chemical atoms, completing three of Sir William Crookes' lemniscates, sufficient for some amount of generalization.- Annie Besant and Charles W. Leadbeater, Occult Chemistry, Clairvoyant Observations on the Chemical Elements (1919) Chapter III. The Later Researches. pp. 18-19.
- Iodine was discovered by Courtois of Paris in 1812 in the mother-liquors collected in the process of manufacturing the sodium salts from kelp or burnt sea-weeds. The name is derived from a Greek word meaning "violet" in allusion to the colour of the vapour of iodine. Its elementary character was established by Gay-Lussac in 1815.
- James Campbell Brown, A History of Chemistry from the Earliest Times (1920) pp.506-507
- In a sense, human flesh is made of stardust.
Every atom in the human body, excluding only the primordial hydrogen atoms, was fashioned in stars that formed, grew old and exploded most violently before the Sun and the Earth came into being. The explosions scattered the heavy elements as a fine dust through space. By the time it made the Sun, the primordial gas of the Milky Way was sufficiently enriched with heavier elements for rocky planets like the Earth to form. And from the rocks atoms escaped for eventual incorporation in living things: carbon, nitrogen, oxygen, phosphorus and sulphur for all living tissue; calcium for bones and teeth; sodium and potassium for the workings of nerves and brains; the iron colouring blood red… and so on.
No other conclusion of modern research testifies more clearly to mankind’s intimate connections with the universe at large and with the cosmic forces at work among the stars.- Nigel Calder, The Key to the Universe (1977)
- I need only remind you of Davy's great researches: nitrous oxide; electric conduction and decomposition—resulting, on the one hand, in the separation of potassium and sodium, the decomposition of the earths following as a necessary consequence, and on the other in the electro-chemical theory; iodine and chlorine—resulting in the extension and confirmation of the word element, the discovery of the so-called hydrogen acids, and the important modification of the French theory of the constitution of acids; the investigation of gaseous explosion and of flame, and the invention of the safety lamp. These are the contributions to science which stand out more prominently in connection with Davy. But over and above all this is the peculiar manner of his discoveries. He was no patient plodder. He did not elaborate his work in minute detail. He dashed it off in broad masses; but just on that account there has never been anyone to follow up his investigations. Davy's mantle fell on no one, not even on Faraday.
- The distinction would only come to Mendeleev halfway through writing his Principles of Chemistry. ...chemical practice and not chemical theory had provided his initial organizing principle... Up to this point [Chapter 20], Mendeleev had only treated four elements in any detail: oxygen, carbon, nitrogen, and hydrogen—the so-called "organogens." Mendeleev began this chapter as usual by purifying the central substance, sodium chloride, from sources such as seawater. A discussion of sodium and chlorine followed in the next few chapters, and finally the halogens appeared... that were closely related to chlorine... and the alkali metals (the sodium family) form the first chapter of volume 2. ...he had dealt with only 8 elements, relegating 55... to the second volume. ...Mendeleev's earlier system of pedalogically useful organization—using laboratory practices... could no longer sustain the burden of exposition. He needed a new system... and he hit upon the idea of using a numerical marker for each element. Atomic weight seemed the most likely candidate for a system that would (a) account for all remaining elements; (b) do so in limited space; and (c) maintain some pedagogical merit. His solution, the periodic system, remains one of the most useful tools in chemistry.
- Michael D. Gordin, A Well-Ordered Thing: Dimitrii Mendeleev and the Shadow of the Periodic Table (2004) p. 22.
- Study of the conduction of electricity in liquids became possible at the beginning of the nineteenth century, following the discovery of the electrolytic cell by Volta in 1800, which provided the first continuous source of electric current. It was soon discovered that the conduction of electricity by solutions is accompanied by chemical reactions at the electrodes which serve to conduct the current into and out of the solution. Nicholson and Carlisle demonstrated the decomposition of water into hydrogen and oxygen by a current in 1801. Davy's discovery of sodium and potassium metals by electrolysis of moist soda and [caustic] potash was a striking example of the novelty of electrochemical decomposition. Many of the phenomena of electrolysis were already known when Michael Faraday began his researches.
- A. Horsfield, "The Faraday and Its Significance in Determining the Fundamental Constants," Precision Measurement and Fundamental Constants; Proceedings (1971) Issue 343, pp. 137-138, U.S. National Bureau of Standards.
- Abnormal sodium metabolism may be critical in the causation of certain forms of hypertension, particularly salt-sensitive hypertension. Long-term restriction of sodium intake in patients at high risk for the development of hypertension may reduce the chances of established hypertension occurring later. These high-risk patients in whom subsequent hypertension may be prevented include normotensive patients with family histories of hypertension, elderly patients, black patients, and those with low-renin hypertension. Treatment of hypertension with moderate sodium restriction to 70 mEq/day will significantly reduce blood pressure in a large percentage of patients, particularly known salt-sensitive hypertensive patients. This degree of restriction is also an effective adjunctive therapy for patients receiving antihypertensive medications. There is convincing experimental, epidemiologic, and clinical evidence that moderate sodium restriction helps prevent and assists in the treatment of hypertension in those patients who are genetically predisposed to develop primary hypertension or who already have hypertension. There is no evidence that this degree of sodium restriction is harmful.
- Mark C. Houston,"Sodium and hypertension. A review" Archives of Internal Medicine (January 1986) 146(1) pp. 179–185, doi:10.1001/archinte.1986.00360130217028.
- [T]he conditions for BEC in alkali gases are reached by combining two cooling methods. Laser cooling is used to precool the gas. The principle of laser cooling is that scattered photons are on average blue-shifted with respect to the incident laser beam. As a result, the scattered light carries away more energy than has been absorbed by the atoms, resulting in net cooling. Blue-shifts are caused by Doppler shifts or ac Stark shifts. The different laser cooling schemes are described in the 1997 Nobel lectures in physics... After the precooling, the atoms are cold enough to be confined in a magnetic trap. Wall-free confinement is necessary, otherwise the atoms would stick to the surface of the container. It is noteworthy that similar magnetic confinement is also used for plasmas which are too hot for any material container. After magnetically trapping the atoms, forced evaporative cooling is applied as the second cooling stage ... In this scheme, the trap depth is reduced, allowing the most energetic atoms to escape while the remainder rethermalize at steadily lower temperatures. Most BEC experiments reach quantum degeneracy between 500 nK and 2 μK, at densities between 1014 and 1015 cm-3. The largest condensates are of 100 million atoms for sodium, and a billion for hydrogen; the smallest are just a few hundred atoms.
- The purpose of cow's milk is to turn a 65-pound calf into a 400-pound cow, as rapidly as possible. Cow's milk is baby calf growth fluid. That's what this stuff is. Everything in that white liquid, the hormones, the lipids, the protein, the sodium, the growth factors, the IGF, every one of those is meant to blow that calf up to a great big cow, or it wouldn’t be there. And whether you pour it onto your cereal as a liquid, whether you clot it into yogurt, whether you ferment it into cheese, whether you freeze it into ice cream, it's baby calf growth fluid. And women eat it and it stimulates their tissues, and it gives women breast lumps, it makes the uterus get big, and they get fibroids and they bleed and they get hysterectomies, and they need mammograms, and it gives guys man-boobs. Cow's milk is the lactation secretions of a large bovine mammal who just had a baby. It's for baby calves. I tell my patients, "Go look in the mirror. Do you have big ears, do you have a tail? Are you a baby calf? If you’re not, don’t be eating baby calf growth fluid." In any level, there’s nothing in it people need.
- Michael Klaper, Interview in the documentary-film Cowspiracy (2014) by Kip Andersen & Keegan Kuhn
- Before we can rightly understand the principles of spectroscopic astronomy, we must go back to the life and work of its founder—Joseph von Fraunhofer. ...Allowing light from the Sun to pass through a prism attached to the telescope, he was amazed to find several dark lines in the spectrum. ...Fraunhofer named the more prominent lines by the letters of the alphabet from A in the red to H in the violet. They are now known as the Fraunhofer lines. ...He expressed the belief that the pair of lines in the solar spectrum which he marked D, coincided with the pair of bright lines emitted by incandescent sodium. Although he doubtless suspected that the lines conveyed intelligence regarding the elements in the Sun, he never was able properly to decipher their meaning. ...Had he lived he would probably have made the great discovery.
- Hector Macpherson, A Century's Progress in Astronomy (1906) p. 47-50.
- Black... set himself the problem of accurately determining the differences in composition between burnt (or caustic) and unburnt (or mild) alkali, and he solved the problem most successfully. He showed that the properties of mild alkalis differ from those of caustic alkalis, because the composition of the former differs from that of the latter; and he showed exactly wherein this difference of composition consists, viz. in the possession or non-possession of fixed air.
Strange we may say that this discovery did not induce Black to prosecute the study of caustic alkalis: surely he would have anticipated Davy, and have been known as the discoverer of potassium and sodium.- M. M. Pattison Muir, Heroes of Science. Chemists. (1883) p. 176.
- When a strong electric current is passed through melted salt, a light, shining, metal-like solid [sodium], and a yellow gas [ chlorine ], having a very penetrating odour, are formed in place of the salt.
- Alkali. (Arabic, al Kali.) A name applied to a well-defined class of bodies characterized by the following properties. They turn red litmus paper blue, completely neutralize acids, they are soluble in water, and their solutions exert a caustic action upon animal matter. The alkalies proper are the oxides of potassium, sodium, rubidium, and cæsium. To these must be added the compound alkali ammonia, the oxide of the hypothetical metal ammonium, which used to be called the volatile alkali, in contradistinction to potash and soda, which were called fixed alkalies. The alkaline earths are the oxides of barium, strontium, calcium, and magnesium. The oxides of some other metals, such as silver, thallium, and lead, are also somewhat soluble in water, and possess slight alkaline properties.
- G. F. Rodwell, ed., A Dictionary of Science; Comprising Astronomy, Chemistry, Dynamics, Electricity, Heat, Hydrodynamics, Hydrostatics, Light, Magnetism, Mechanics, Meteorology, Pneumatics, Sound, and Statics; Preceded by an Essay on the History of the Physical Sciences (1873) p. 41.
- Dumas devised an accurate and excellent method for determining the specific gravities, or densities, of gases which could be used at high temperatures, thus enabling him to experiment upon the vapor densities of iodine, phosphorus, sulphur, mercury, etc. His results, instead of confirming, tended rather to disprove the law of volumes. The trouble lay in the complex nature of the molecules experimented upon, but of course this was unknown to Dumas. He finally declared that even in the case of the simple gases like volumes did not contain equal numbers of chemical atoms. ...The atomic weights determined by him with the greatest care were those of silver, potassium, sodium, lithium, lead, chlorine, bromine, iodine, sulphur, and nitrogen.
- Francis Preston Venable, History of Chemistry (1922) pp.75-77
- The first numerical regularities observed between the atomic weights were the triads of Döbereiner. This chemist seems to have observed first that the combining weight of strontium was the arithmetical mean of those of calcium and barium. A like regularity was noted with regard to certain physical properties of these elements and some of their compounds. This led him for a while to question the independent existence of strontium. Several similar triads were discovered among the other elements as lithium, sodium, and potassium; chlorine, bromine, and iodine; sulphur, selenium, and tellurium. He was careful not to let this grouping depend upon the atomic weights alone but insisted that only elements exhibiting decided analogies of properties should be considered together. This idea was taken up by other chemists, notably by Gmelin in his Handbook, and many analogies and groups were sought for. In 1857 [Ernst] Lennsen returned to this grouping, endeavoring to force all the elements into some twenty groups. Then Odling sought to build upon them an elaborate system of the elements which he called the Natural System. Such groupings were often forced, and failures. The science was not far enough advanced to enable one to understand the real meaning of these regularities.
- Francis Preston Venable, History of Chemistry (1922) pp.83-84.
- If you pass the light from a sodium flash through a prism, you get a pattern very different from the familiar continuous rainbow that Newton elicited from natural sunlight. Instead of a continuous pattern, in which all gradations of pure color are apparently represented, the sodium flash generates a series of lines of light. ...in the musical analogy, sodium produces a chord where sunlight produced all possible tones—"white noise." Other elements produce other chords.
- Frank Wilczek, Betsy Devine, Longing for the Harmonies: Themes and Variations from Modern Physics (1987)
Chemical Analysis by Spectral Observations (1860)
[edit]- by Gustav Kirchhoff and Robert Bunsen, Annalen der Physik (1860) ed., Johann Christian Poggendorff, Vol. 110, 1860. pp. 275-301. As quoted in The Laws of Radiation and Absorption: Memoirs by Prévost, Stewart, Kirchhoff, and Kirchhoff and Bunsen (1901) Tr., ed., DeWitt Bristol Brace, pp. 107-109. See also Kirchhoff and Bunsen, "Chemical Analysis by Spectrum-observations" (Aug, 1860) The London, Edinburgh and Dublin Philosophical Magazine and Journal of Science, pp. 94-96.

- We will now consider more closely the characteristics of the several spectra, the knowledge of which is of importance from a practical standpoint, and indicate the advantage which the chemical analytical method founded upon it furnishes.

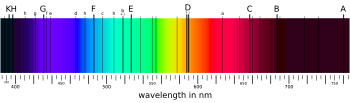
Solar spectrum

- SODIUM. Of all the spectral reactions that of sodium is the most sensitive. The yellow line Naα, the only one which is shown in the sodium spectrum coincides with Fraunhofer's line D and is characterized by its peculiarly sharp boundary and its extraordinary brilliancy. If the temperature of the flame is very high and the quantity of the substance used very great, traces of a continuous spectrum are seen in the immediate neighborhood of the line.
- Lines of other substances, in themselves very weak, lying near it appear still weak and will, therefore, often first be visible after the sodium reaction has begun to disappear.
- Swan has already called attention to the minuteness of the quantity of common salt which can produce the sodium line clearly.
- Ref: Transactions of the Royal Society of Edinburgh, Vol XXI, Part III, p. 411. Also see Swan, Pogg. Anu. Vol. C, p. 311.
- The following investigation shows that chemistry produces no single reaction which in the remotest degree can compare in sensitiveness with this analytical spectral determination of sodium.
- We detonized in one corner of the experiment room which contained about 60 cubic meters of air and as far as possible from our apparatus three milligrams of chlorate of sodium with milk sugar while the non-luminous flame was observed before the slit. After some minutes, the flame, becoming gradually colored pale yellow, gave a strong sodium line, which, after ten minutes, again completely vanished.
- From the weight of the detonized salt and the air contained in the room it is easy to calculate that in a unit weight of the latter not a th part of sodium smoke could have been suspended. ...[I]t follows that the eye is capable of detecting less than th of a milligram of sodium salt with the greatest distinctness. With such a sensibility of the reaction it is evident that only rarely is a sodium reaction not visible in glowing atmospheric air.
- The earth is covered over more than two-thirds of its surface with a solution of chloride of sodium, which, by the waves breaking into foam, is transformed continually into spray; the particles of sea-water, which reach the atmosphere in this way, evaporate and leave behind them motes of salt which vary in magnitude, but, as it appears, are rarely absent from the atmosphere, and, perhaps, serve to supply the small organisms the salt which the larger plants and animals secure from the ground.
- If, as we yet can scarcely doubt, there are catalytic influences which are the cause of the miasmic spread of disease, it is possible that an antiseptic substance, such as salt, even in vanishingly small quantities, may indeed not be without definite influence upon such processes in the air. From daily and long continued spectrum observation it would be easy to learn whether the variation in the intensity of the spectral line Naα, produced by the sodium combination in the air, is related in any degree to the appearance and the spread of endemic diseases.
- In the exceedingly delicate sodium reaction may also be sought the reason why all bodies exposed to the air show the sodium line after a time when heated in the flame, and why it is possible with only a few compounds to eliminate the last trace of the sodium line Naα by crystallizing it out ten or more times from water which has come in contact with platinum vessels only.
- A hair wire of platinum, which has been freed, by heating, from every trace of sodium, shows the reaction most vividly again, if it is exposed some hours to the air. Dust which settles in the room from the air shows it in the same degree, so that, for example, the slapping of a dusty book is quite sufficient to produce at a distance of several spaces the most brilliant flashes of the Naα line.
The Principles of Chemistry (1891)
[edit]- by Dmitri Mendeleev, Tr. George Kamensky, of Основы химии (1867) See also 2 Volume (1905) edition.
- Mercury as a liquid metal is capable of dissolving other metals and forming metallic solutions. These are generally called 'amalgams.' The formation of these solutions is often accompanied by the development of a large amount of heat—for instance when potassium and sodium are dissolved... but sometimes heat is absorbed, as... when lead is dissolved. It is evident that phenomena of this kind are exceedingly similar to the phenomena accompanying the dissolution of salts and other substances in water, but here it is easy to demonstrate that which is far more difficult to observe in the case of salts: the solution of metals in mercury is accompanied by the formation of definite chemical compounds of the mercury with the metals dissolved. This is shown by the fact that when pressed (best of all in chamois leather) such solutions leave solid, definite compounds of mercury with metals. It is, however, very difficult to obtain them in a pure state, on account of the difficulty of separating the last traces of mercury, which is mechanically distributed between the crystals of the compounds. Nevertheless, in many cases such compounds have undoubtedly been obtained, and their existence is clearly shown by the evident crystalline structure and characteristic appearance of many amalgams.
- [T]he sulphate of sodium prepared at chemical works is converted into soda ash—that is, sodium carbonate, Na2CO3 , which is used for many purposes. In the form of carbonates, the metallic oxides behave in many cases just as they do in the state of oxides or hydroxides, owing to the feeble acid properties of carbonic acid. However, the majority of the salts of carbonic acid are insoluble, whilst sodium carbonate is one of the few soluble salts of this acid and therefore reacts with facility. Hence sodium carbonate is employed for many purposes, in which its alkaline properties come into play. Thus, even under the action of feeble organic acids it immediately parts with its carbonic acid, and gives a sodium salt of the acid taken. Its solutions exhibit an alkaline reaction on litmus. It aids the passage of certain organic substances (tar, acids) into solution, and is therefore used, like caustic alkalies and soap (which latter also acts by virtue of the alkali it contains), for the removal of certain organic substances, especially in bleaching cotton and similar fabrics.
- [A] considerable quantity of sodium carbonate is used for the preparation of sodium hydroxide or caustic soda, which has also a very wide application. In large chemical works where sodium carbonate is manufactured from Na2SO4, it is usual first to manufacture sulphuric acid, and then by its aid to convert common salt into sodium sulphate, and lastly to convert the sodium sulphate thus obtained into carbonate and caustic soda. Hence these works prepare both alkaline substances (soda ash and caustic soda) and acid substances (sulphuric and hydrochloric acids), the two classes of chemical products which are distinguished for the greatest energy of their reactions, and are therefore most frequently applied to technical purposes. Factories manufacturing soda in this manner are generally called alkali works.
- Pure sodium is a lustrous metal, at the ordinary temperature as white as silver and as soft as wax, but it becomes brittle in the cold. In ordinary moist air it quickly tarnishes and becomes covered with a film of NaHO and Na2CO3, formed at the expense of the water and CO2 in the air. In perfectly dry air sodium retains its lustre for an indefinite time. Its density at the ordinary temperature is equal to 0.975, so that it is lighter than water; it fuses very easily at a temperature of 97°, and distils at a bright red heat (742°, according to Perman, 1889). Scott (1887) determined the density of sodium vapour and found it to be nearly 12 (if H = 1). This shows that its molecule contains one atom (like mercury and cadmium), Na. It fuses with most metals, forming indefinite compounds called alloys. Thus, if sodium, having a clean surface[,] be thrown into mercury, especially when heated, there is a flash, and such a considerable amount of heat is evolved that part of the mercury is transformed into vapour. Compounds or solutions of sodium in mercury, or amalgams of sodium, even when containing only 2 parts of sodium to 100 parts of mercury, are solid. Only those amalgams which are the very poorest in sodium are liquid. Such alloys of sodium with mercury are often used instead of sodium in chemical investigations, because in combination with mercury sodium is not easily acted on by air, and is heavier than water, whilst at the same time it retains its principal properties, such, for instance, as the power to decompose water, forming NaHO.
- The most important chemical property of sodium is its power of easily decomposing water and evolving hydrogen from the majority of the hydrogen compounds, and especially from all acids and hydrates in which the presence of hydroxyl must be recognised. This depends on its power of combining with the elements which are in combination with the hydrogen. ...[S]odium disengages hydrogen, not only from water, hydrochloric acid, and all other acids, but also from ammonia, with the formation of sodamide, NH2Na, although it does not displace hydrogen from the hydrocarbons. Sodium burns both in chlorine and in oxygen, evolving much heat. These properties are closely connected with its power of taking up oxygen, chlorine, and similar elements from most of their compounds.
Humphry Davy, Poet and Philosopher (1896)
[edit]- Main article: Humphry Davy, Poet and Philosopher (Thorpe)
Note: quotes are from Ch. VI. The Isolation of the Metals of the Alkalis. unless otherwise noted.
- It would seem from his description of its properties that the potassium he obtained was most probably alloyed with sodium derived from impure potash. Potassium is solid up to 143° F.; but, as Davy subsequently found, an alloy of potassium and sodium is fluid at ordinary temperatures.
- The "basis" of soda is described as a white opaque substance of the lustre and general appearance of silver. It is soft and malleable, and is a good conductor of heat and electricity. Its specific gravity was found by flotation in a mixture of oil of sassafras and naphtha... It was found to fuse at about 180° F. (the real melting point of sodium is 197.5°). Its action on a number of substances—oxygen, hydrogen, water, etc.—is then described, and its general behaviour contrasted with that of the "basis" of potash.
- He then enters upon some general observations on the relations of the "bases" of potash and soda to other bodies.
- "Should the bases of potash and soda be called metals? The greater number of philosophical persons to whom this question has been put, have answered in the affirmative. They agree with metals in opacity, lustre, malleability, conducting powers as to heat and electricity, and in their qualities of chemical combination."
- "Their low specific gravity does not appear a sufficient reason for making them a new class; for amongst the metals themselves there are remarkable differences in this respect, and in the philosophical division of the classes of bodies, the analogy between the greater number of properties must always be the foundation of arrangement."
- "On this idea, in naming the bases of potash and soda, it will be proper to adopt the termination which, by common consent, has been applied to other newly discovered metals, and which, though originally Latin, is now naturalized in our language."
- "Potasium [sic] and sodium are the names by which I have ventured to call the new substances; and whatever changes of theory, with regard to the composition of bodies, may hereafter take place, these terms can scarcely express an error; for they may be considered as implying simply the metals produced from potash and soda. I have consulted with many of the most eminent scientific persons in this country, upon the methods of derivation, and the one I have adopted has been the one most generally approved. It is perhaps more significant than elegant. But it was not possible to found names upon specific properties not common to both; and though a name for the basis of soda might have been borrowed from the Greek, yet an analogous one could not have been applied to that of potash, for the ancients do not seem to have distinguished between the two alkalies."
- He begins by again drawing attention to the various surmises which had been made respecting the true nature of potassium and sodium. Although these substances had been isolated, and in the hands of chemists for upwards of two years, their properties were so extraordinary when compared with those of the metals in general, that many philosophers hesitated to consider them as true metals.
"The Development of the Electron Idea" (Nov. 8, 1901)
[edit]- Walter Kaufmann, The Electrician Vol. 48, pp. 95-97. Lecture delivered before the 73rd Naturforscher Versammlung at Hamburg. From the Physikalische Zeitshrift, of October 1, 1901.
- [A]lthough the plans of the edifice of the electromagnetic theory of light were laid in 1880 by H. A. Lorentz, and even indicated much earlier by W. Weber, a full 10 years were required before the discoveries of Heinrich Hertz gave the impetus to collect the building stones and work them into shape. In the years 1890-93 a number of works appeared by F. Richarz, H. Ebert and G. Johnstone Stoney, mostly dealing with the mechanism of the emission of luminous vapours, and in which attempts are made, on the basis of the kinetic theory of gases, to determine the magnitude of the elementary electrical quantity, called by Stoney by the now universally accepted name of electron. ...H. Ebert proved that the amplitude of an electron in luminous sodium vapour need only be a small fraction of a molecular diameter in order to excite a radiation of the absolute intensity determined by E. Wiedemann. The way of determining the amount of electricity contained in the electron is very simple. The quantity of electricity required for the electrolytic evolution of 1 cubic cm. of any monatomic gas is divided by Loschmidt's number—i.e., the number of gas molecules contained in 1 cubic cm.
- In view of the facility with which Lorentz's theory explains the dispersion and observation phenomena, a direct proof of its truth was hardly required. But that was also forthcoming. In 1896 a pupil of Lorentz, P. Zeeman, discovered a phenomenon whose existence Faraday had vainly sought for in 1862. If a luminous vapour, say a sodium flame, is brought into a strong magnetic field, the spectrum lines of the vapour show peculiar changes, consisting of a doubling or trebling, according to the line of vision. These changes are predicted by Lorentz's theory. The Zeeman phenomenon further permitted a determination of the inert mass connected with the vibrating charges, and then a striking result was obtained: the vibrating electron is always negatively charged, while the positive charge is stationary. ...The original and almost tacit assumption that the whole ion—i.e., the chemical atom plus its valency charge—was in oscillation must, therefore, be abandoned. We must suppose that the charge, just as is the case in electrolysis, has also an independent mobility in the light-emitting molecule, and that the mass concerned in the Zeeman phenomenon is that of the electron itself.
The Centenary of Davy's Discovery of the Metals of the Alkalis (1908)
[edit]- by Thomas Edward Thorpe. A Discourse delivered before the Royal Institution , January 17, 1908, as quoted in The Chemical News and Journal of Physical Science Vol. 97, No. 2527, pp. 210-211. (May 1, 1908)
- The publication of Davy's discovery created an extrordinary sensation throughout the civilised world, a sensation not less profound, and certainly more general from its very nature, than that which attended his lecture of the previous year. But at the very moment of his triumph, it seemed that the noise of the universal acclaim with which it was received was not to reach him.
- Ref: Humphry Davy, The Bakerian Lecture, on some new Phenomena of chemical Changes produced by Electricity, particularly the Decomposition of the fixed Alkalis, and on the Exhibition of the new substances which constitute their bases; and on the general Nature of alkaline Bodies (Read Nov 9, 1807) Philosophical Transactions of the Royal Society of London (1808) Part. I, pp. 1-44.
- Have sodium and potassium at all justified the hope that they would facilitate the means of procuring the comforts and conveniences of life? I have not the time... to attempt to follow the many changes in the metallurgy of the metals of the alkalis of the past century. Let me... show how the matter stands at the end of a hundred years.
- The general properties and chemical activities of potassium and sodium are so very similar that as a matter of commercial production that metal which can be most economically obtained is necessarily the one most largely manufactured, and of the two that metal is sodium. To-day, sodium is made by thousands of tons, and by a process which in principle is identical with that by which it was first made by Davy, i.e., by the electrolysis of fused caustic soda.
- [A]fter a series of revolutions in its manufacture, sodium, having been produced from time to time on a manufacturing scale by a variety of metallurgical methods involving purely thermal processes of reduction and distillation, entirely dissociated from electricity, we should have now got back to the very principle of the process which first brought the metal to light. And that this has been industrially possible is entirely owing to another of Davy's discoveries - possibly indeed the greatest of them all—Michael Faraday.
- As we all gratefully acknowledge, it is to the genius and labours of Faraday—Davy's successor in this place—that the astonishing development of the application of electrical energy which characterises this age has taken rise.
- The modern method of production of sodium is based, therefore, as regards principles upon the conjoint labours of Davy and Faraday.
- These principles took their present form of application at the hands of... Hamilton Y. Castner... It is by Castner's process that all the sodium of to-day is manufactured.
In the Castner process melted caustic soda produced by the electrolysis of a solution of common salt by a method also devised by Castner, is brought into an iron vessel shaped like a large cauldron, mounted in brickwork, and provided with an extension adapted to receive the negative electrode. Suspended directly above the cathode is an iron vessel attached to a lid; to its lower edge is secured iron-wire gauze, which, when the receptacle is in position, completely surrounds the cathode. The positive electrode is connected with the lid of the vessel, which is provided with openings for the escape of the gases resulting from the electrolysis, and is suitably insulated.
- As the electrolysis proceeds the alkali metal, being much lighter than the molten caustic, rises from the negative electrode and passes into the receiver, the gases escaping around the edges of the cover. The molten metal collects on the surface of the caustic, and is removed by means of a large perforated spoon, the perforations enabling the melted caustic to flow out, while the metal remains in the spoon. As the several vessels are thus skimmed in succession the fused sodium is collected into an iron vessel, whence it is poured into moulds in which it congeals, forming blocks of the size and shape of an ordinary building brick. These, after being trimmed to remove adherent oxide, are immersed in paraffin oil, and are then packed into large iron drums... capable of being closed air-tight, and protected in transit by an outer casing of wood.
- The due regulation of the volume and intensity of the current is a matter of the greatest importance in order to obtain the most economical yield of the metal. No very high temperature is needed; indeed, the temperature of the fused caustic soda should not be much higher than that of its melting point. By suitably regulating the current, the soda, in fact, may be maintained at the proper temperature and in the proper degree of fluidity without extraneous heat. Fresh melted caustic soda is added to the vessel from time to time to replace the metal removed, and in this manner the process is made continuous.
- The greater quantity of the sodium made in England is... converted into sodium cyanide... for use in the extraction of gold. As gold is... generally considered the principal material factor in procuring the comforts and conveniences of life, Davy's great discovery may be thus said to have secured the primary object which the projectors of the Royal Institution had in view. Other important uses of sodium are in the manufacture of peroxide for bleaching purposes, of artificial indigo, and of a number of other synthetic dye stuffs and of drugs like antipyrin.
- [T]his extraordinary development of the manufacture has not been without its influence on the price of sodium. A quarter of a century ago it was a comparatively rare metal, and a stick of it was regarded as a chemical curiosity, to be handled with circumspection and care. Even as late as 1890 its selling price was as high as 8s. per lb. To-day it is 8d. Sodium now takes rank, therefore, with zinc, tin, copper, or aluminium as a common, ordinary metal of commerce.
- I am indebted to the directors of the Castner-Kellner Company... or affording me the opportunity, in connection with this lecture, of actually witnessing the modern process of manufacturing sodium as it is carried out at Wallsend... And in concluding may I be permitted to recall here the feelings to which that visit to Wallsend gave rise. ...Before me, stretching down to the river, was the factory where a score of workers, clad in helmets and gauntlets and swathed like so many Knights Templar, travel-stained and war-worn, their visages lit up by the yellow soda flames, and their ears half-deafened with the sound of exploding hydrogen—a veritable inferno—were repeating on a Gargantuan scale the little experiment first made a century ago in the cellars of this building; turning out, day and night, hundredweights of the plastic metal in place of the little pin-heads which then burst upon the astonished and delighted gaze of Davy.
- Behind me was the magnificent power-house... furnishing not only the electrical energy which transformed the soda into sodium, but diffusing this energy for a multitude of other purposes over an entire district—a noble temple to the genius and prescience of Faraday. Surely one might here say, if you desire to see the monuments of these men, look around!
The Mind (1984)
[edit]
- The composition of every cell is different from that of the material in which it exists. ...The striking feature of a neuron at rest ...its contents are one-tenth as rich in sodium ions as the external fluid and... ten times richer in potassium ions. ...[S]odium ions will leak into the cell and potassium will leak from it. ...[T]he membrane possesses pumps to offset the flow ...sodium-potassium adenosine triphosphotase pumps ...can steadily exchange three sodium ions for two potassium ...Every neuron contains about a million pumps... and every pump can swap about 200 sodium ions for 130 potassium ions every second.. ...The pumps ...maintain that sodium potassium imbalance, and... the inside of each neuron at seventy millivolts negative to the outside. ...[T]hat helps to explain the near-permanent demand for oxygen ...
- [A] change in the electrical state of the nerve cell... hurries along the axon in advance of the impulse... partnered by an alteration in the permeability of the membrane... permitting sodium ions to pour into the cell... [T]he more... ions rush in the more channels are opened to increase this torrent. Suddenly, with a change in the membrane potential from negative to positive... sodium channels close. Another group... opens, permitting potassium ions to flow out... until the... minus seventy millivolts is achieved again.
- The transmission of an impulse is not electrical... The so-called spike... the shift in membrane potential between -70 millivolts and +40 millivolts, is the electrical manifestation of each nerve impulse. ...[T]here is plainly more to it—with channels opening, pumps operating, and sodium scurrying back and forth—than... an electrical impulse along a copper wire.
- [C]onsiderable attention has been paid to the 'blood-brain barrier'. ...[I]n 1909 ...it was realized that certain substances, such as dyes, did not reach the brain. ...[T]hey arrived swiftly within every other tissue but not the brain or spinal cord. ...There was apparent restriction both to foreign substances (sucrose, insulin, penicillin) and natural ones (urea, sodium, potassium, creatinine). By comparison with muscular tissue, where there is equilibrium between blood and tissue for the injected substance within seconds or minutes, such a balance in the brain may take hours.
See also
[edit]External links
[edit]- Sodium at Periodic Videos (University of Nottingham)
- Etymology of "natrium" and of the symbol Na.
- Na, Sodium, The Wooden Periodic Table.
- Isotopes of Sodium (Z=11) The Berkeley Laboratory Isotopes Project from the Wayback Machine @archive.org.



