Influenza vaccine
Appearance
Influenza vaccines, also known as flu jabs or flu shots, are vaccines that protect against infection by influenza viruses.
Quotes
[edit]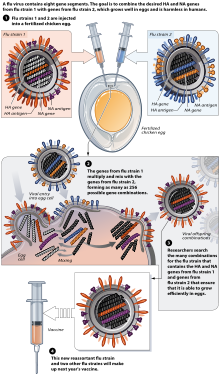

- There are very few, if any, actual scientific studies that have been performed on vaccine safety. Not for any vaccine, not for any age group of recipients.
- Theodore Beale, There Are No Studies, Vox Day, 11 July 2024
- The use of licensed inactivated trivalent influenza vaccine is increasing, but even if all high risk persons currently given priority for this vaccine should be vaccinated each year, influenza epidemics would continue to occur.
- Glezen W Paul. “Emerging infections: pandemic influenza”. Epidemiol Rev. 1996;18(1): p.73
- Recent pandemics illustrate another problem that must be faced with an impending pandemic. The time between recognition of the emergence of a new pandemic virus and the occurrence of the first wave may be short. The lead time for the production and distribution of the currently licensed influenza vaccine, trivalent influenza vaccine is 6 months. It is highly unlikely that sufficient vaccine can be produced, distributed and administered to the entire population before the first wave of the pandemic. In 1918 and 1957, the first wave of the pandemic peaked in late October allowing less time than usually occurs before the onset of interpandemic outbreaks, in the usual sequence of vaccine production starting in January.
- Glezen W Paul. “Emerging infections: pandemic influenza”. Epidemiol Rev. 1996;18(1): p.73
- In the last 100 years, numerous vaccines have become available for influenza prevention. In the United States, national vaccine policy recommends influenza vaccination annually for everyone older than age 6 months. There are multiple types of vaccine that use different inactivated, live-attenuated, and egg-free formulations. Recent efforts through WHO’s Global Action Plan for Influenza Vaccines and the Pandemic Influenza Preparedness framework supported efforts to increase vaccine manufacturing and laboratory capacity for identifying viruses for use in vaccines. The global pandemic influenza vaccine production capacity in 2015 was estimated to be 6.4 billion doses—a record level, but not enough to provide the potential need for 2 doses for even half the world’s population. The current timeline for vaccine production also limits the usefulness of pandemic vaccine, as reflected in 2009, when the bulk of pandemic vaccine was not available until after the peak of the pandemic. However, increased use of new vaccine formulations that do not rely on growing viruses in eggs, such as cell-based vaccine and recombinant protein vaccine, will reduce the time required for vaccine manufacturing. In addition, further expansion of seasonal influenza vaccine manufacturing capacity worldwide, and continued increases in use of vaccine, will facilitate pandemic vaccine production and global access to pandemic vaccines.
- Jester, B; Uyeki, T; Jernigan, D. “Readiness for Responding to a Severe Pandemic 100 Years After 1918”: American Journal of Epidemiology. Volume 187, Issue 12, December 2018, pp.2596–2602
- Many challenges remain to improving influenza vaccines. Current seasonal influenza vaccines, at best, are only moderately effective in preventing illness and often have low effectiveness. Greater monitoring of vaccine effectiveness is needed to better inform incremental improvements in current influenza vaccines. A more broadly protective and longer-lasting (i.e., “universal”) vaccine could decrease the current need for frequent formulation change and improve prevention of influenza worldwide, especially in low-resource and middle-income countries. Rapid development of vaccine candidates, accelerated clinical trials, and reducing the time required to formulate and distribute pandemic vaccine can reduce pandemic morbidity and deaths. Therefore, reducing the current pandemic influenza vaccine availability timeframe from 20 to 12 weeks is a key priority in the 2017 Update of the Health and Human Services Pandemic Influenza Plan.
- Jester, B; Uyeki, T; Jernigan, D. “Readiness for Responding to a Severe Pandemic 100 Years After 1918”: American Journal of Epidemiology. Volume 187, Issue 12, December 2018, pp.2596–2602
- Despite our modern arsenal of antibiotics, of viral and bacterial vaccines, of antiviral drugs, advanced intensive care treatment, and nonpharmaceutical interventions, we are still doing a poor job of preventing influenza deaths. The most important lesson from the devastation of the 1918 pandemic may be the need to produce better antiviral drugs and prophylactic and therapeutic monoclonal antibody therapies. We need effective vaccines against multiple bacterial pneumopathogens, especially S. aureus and S. pyogenes, and effective broadly protective “universal” influenza vaccines to prevent, or at least mitigate the impact of, future pandemics and to prevent deaths from seasonal influenza in the periods in between pandemics. Vaccines that could confer long-term broad immune responses against all influenza viruses, and especially against viruses with the most pathogenic HAs found in nature, would greatly enhance public health preparedness.
- Jeffery K. Taubenberger et al., “The 1918 influenza pandemic: 100 years of questions answered and unanswered”, Science Translational Medicine, 24 Jul 2019: Vol. 11, Issue 502
“Vaccines for Pandemic Threats”, Histoyofcavvines.org
[edit]
- In the case of an influenza pandemic, existing vaccines would likely be ineffective against a radically new strain. Developing and globally distributing a new pandemic influenza vaccine will likely take 4-6 months (4 months to produce first doses of vaccine, and 6 months to produce enough to give to a large number of people), even while mathematical models demonstrate that pandemic influenza could spread globally within 6 months.
Another complicating factor to pandemic influenza vaccine production involves how the vaccine is made. Since the 1940s, seasonal and pandemic influenza vaccines have been produced in chicken eggs. The virus is introduced in the allantoic fluid of the fertilized egg (this is the fluid that bathes the embryo and yolk sac), and it replicates in the membrane surround the fluid. After about three days, the virus-containing fluid is harvested from each egg, and the rest of the manufacturing process proceeds. Dependence on egg-based vaccine production is, however, problematic even with non-pandemic seasonal influenza vaccine. First, eggs must be available in large quantities when vaccine production is to begin. Any disruption in egg supply – such as disease affecting chickens, or bad weather interfering with the shipping of eggs – can mean a delay in vaccine production. Second, some influenza strains grow more slowly or less robustly than others, which can result in delays or in lower yields of vaccine virus from each egg. Third, it is possible that some viral vaccine strains, given the origin of some influenza viruses in birds, may be toxic to eggs. In that case, egg-based influenza vaccine production methods would be useless. - Production capacity is another limitation to deployment of pandemic influenza vaccine. Current global capacity for pandemic influenza vaccine production is less than 3 billion doses per year, far short of the 7 billion doses that would be needed for universal coverage.
To address some of these problems with egg-based vaccine production, some pharmaceutical companies are attempting to eliminate eggs from the process altogether. Novartis produces influenza vaccine from virus cultivated in cells derived from canine kidney cells (see the FDA information on this vaccine). Protein Sciences Corporation produces influenza vaccine using recombinant DNA technology and an insect virus system (see the FDA information). Other companies are developing influenza vaccine produced from different types of cell lines.
Given that it takes roughly the same amount of time for influenza virus to replicate in eggs and in cell culture, shifting to cell culture will not necessarily speed up this phase of production. However, using cell culture technology will eliminate the lead time necessary to secure fertilized eggs for vaccine production and will reduce some of the variables related to the quantity of vaccine virus achieved with eggs. Additionally, egg-based influenza vaccine production requires a step in which the influenza virus is altered so that it reproduces well in eggs. If cell-based manufacturers can skip that step, they can begin vaccine production 4-6 weeks earlier than egg-based manufacturers. - Other approaches to accelerating influenza vaccine production involve use of what are referred to as dose-sparing technologies. These are innovations that allow less antigen to be used for each vaccine dose, without compromising immunogenicity or safety. Dose-sparing technologies have the potential to markedly increase vaccine production potential in a pandemic. Adjuvants (compounds that enhance the immune response to a vaccine and therefore reduce the amount of vaccine virus required for each dose) are one such technology. The most commonly used adjuvant today is an aluminum compound found in many childhood vaccines, but which is not used in influenza vaccine. Oil-in-water emulsion adjuvants show the greatest advancement and promise in terms of dose sparing for influenza vaccines. Other potential technologies might involve self-adjuvanting recombinant or molecular vaccines that have built-in antigen-sparing properties.
Other promising candidates and technologies may emerge that lead to development of a universal influenza vaccine, which is the ultimate goal for many influenza vaccine programs. Such a vaccine might need to be given only once, rather than every year as with current seasonal vaccines. Such a universal vaccine would ideally provide protection against all, or at least most, of the many strains of influenza capable of making people sick, including future pandemic influenzas. Plant-produced influenza vaccines are in clinical trials and may prove to be a useful alternative to egg- and cell-based vaccines.

