Swine influenza
Appearance

Swine influenza is an infection of a host animal by any one of several specific types of swine influenza virus. In 2009 the media labeled as "swine flu" a disease caused by 2009's new strain of swine-origin A/H1N1 pandemic virus, which is transmitted between humans.
Quotes
[edit]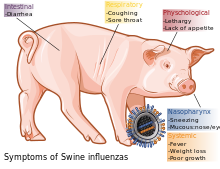
| This theme article is a stub. You can help out with Wikiquote by expanding it! |
- I would tell members of my family -- and I have -- I wouldn't go anywhere in confined places now....It's not (just) going to Mexico, it's you're in a confined aircraft when one person sneezes it goes all the way through the aircraft.
- Joseph Biden, "Biden on swine flu: Mitigation works best", Market Watch, (April 30, 2009).
- I mean, by the fall of 2009, when Joe Biden was vice president the swine flu had infected 60 million Americans, and if it had had the same fatality rate as the coronavirus does, we would have lost more than two million American lives.
- Mike Pence, "Pence defends Trump COVID-19 response, claims administration 'literally saved hundreds of thousands", FOX NEWS FLASH, (October 25, 2020).
- "To a patient whose lungs are temporarily compromised, short-term ventilator support is often the difference between life and death. Unfortunately, if a future pandemic leaves millions of Americans temporarily unable to breath -- vastly exceeding the number of mechanical ventilation machines or the trained staff needed to operate them -- our hospitals will be forced to decide which patients to help breathe and which to let die."
- Jacob M. Appel, physician, The Coming Ethical Crisis: Oxygen Rationing, Huffington Post, June 27, 2009.
- An instructive example of our inability to predict pandemic emergence, or to precisely characterize pandemic viral genetic evolutionary pathways, is that of the 2009 swine influenza pandemic caused by the H1N1pdm virus. This virus appeared in an era of unprecedented human and animal influenza viral surveillance and the near real-time deposition of thousands of influenza virus genome sequences into public databases. The first recognized human cases caused by the 2009 pandemic H1N1pdm virus occurred in Mexico. However, multiple co-circulating genotypes of related swine influenza A viruses, resulting from multiple complex reassortments of various swine influenza A virus lineages (including those similar to the 1918 pandemic H1N1 virus from which the ancestral 2009 swine virus lineage had been derived in 1918), were identified not only in swine populations in central Mexico but also in Asia. Clearly, most, if not all, of the major pre-emergence genetic events of 2009 had happened at some time, and in some unknown place, that escaped detection.
Even if the future pandemic virus had been identified in swine populations in the months and years before 2009, it would likely not have been recognized as a virus with pandemic potential. This is because viral phenotypic properties associated with human adaptation and transmissibility cannot yet be predicted from genetic sequences. The implications are sobering: Identifying pre-pandemic viruses by increased viral surveillance in mammals and birds may be difficult or impossible. There is reason to believe that every influenza virus pandemic and panzootic/epizootic event (the animal counterparts to pandemics and epidemics in humans) may be fundamentally different from every other. Although these pandemic (or panzootic/epizootic) viruses-to-be already exist in nature, or evolve and adapt in humans or other mammals, there is a growing realization that each seems to achieve its host switch success through different cooperative polygenic adaptive mutations that, in unique combination, are able to support pandemic or panzootic spread.- Jeffery K. Taubenberger et al., “The 1918 influenza pandemic: 100 years of questions answered and unanswered”, Science Translational Medicine, 24 Jul 2019: Vol. 11, Issue 502
- A unique epidemiological feature of the 1918 influenza virus, related to its origin, was infection of both humans and swine. Influenza was first recognized as a clinical entity in swine in the United States in autumn 1918, concurrent with the spread of the pandemic in humans, having apparently been transmitted from humans to pigs. This host switch split the virus off into two independent viral lineages, one human and the other porcine. After 1918, the epizootic disease became widespread among herds of swine in the U.S. midwest. Epizootic viruses appeared annually thereafter, leading to Shope’s 1930 isolation of the first influenza virus, A/swine/Iowa/30, 3 years before the first human isolation of a descendant of the parent 1918 virus, A/WS/33. The two 1918 viral H1N1 lineages, one human and the other porcine, evolved and antigenically drifted at different rates until 2009. In the 2009 pandemic, the human-adapted H1N1 descendant was replaced by a different H1N1 virus that was also a 1918 viral descendant, ironically one that had been circulating enzootically in pigs. The original 1918 classical swine lineage still circulates enzootically today.
- Jeffery K. Taubenberger et al., “The 1918 influenza pandemic: 100 years of questions answered and unanswered”, Science Translational Medicine, 24 Jul 2019: Vol. 11, Issue 502
“Case fatality risk of influenza A (H1N1pdm09): a systematic review.” (2013)
[edit]Jessica Y. Wong, Heath Kelly, Dennis K. M. Ip, Joseph T. Wu, Gabriel M. Leung, and Benjamin J. Cowling; “Case fatality risk of influenza A (H1N1pdm09): a systematic review.”, Epidemiology 24, 830–841 (2013).

- In April 2009 the World Health Organization declared a formal “public health emergency of international concern,” marking the start of an international public health response to the first influenza pandemic of the 21st Century. One of the immediate priorities was to quantify the transmissibility of the new pandemic influenza A (H1N1pdm09) virus (denoted H1N1pdm09 hereafter) and the seriousness of infection with this virus, because these two epidemiologic measures in combination determine the severity of the pandemic in the absence of control measures. Whereas a number of transmissibility estimates, based on the reproduction number R, were published with broad agreement from the early stages of the pandemic, there was far greater difficulty in estimating the seriousness of infections. In the report of the World Health Organization’s Review Committee on the functioning of the 2005 International Health Regulations in relation to H1N1pdm09, Fineberg et al. identified “the absence of a consistent, measurable and understandable depiction of severity of the pandemic” as one of the major shortcomings of the international public health response.
- There is very substantial heterogeneity in published estimates of case fatality risk for H1N1pdm09, ranging from <1 to >10,000 per 100,000 infections. Large differences were associated with the choice of case definition (denominator). Because influenza virus infections are typically mild and self-limiting, and a substantial proportion of infections are subclinical and do not require medical attention, it is challenging to enumerate all symptomatic cases or infections. In 2009, some of the earliest available information on fatality risk was provided by estimates based primarily on confirmed cases. However, because most H1N1pdm09 infections were not laboratory-confirmed, the estimates based on confirmed cases were up to 500 times higher than those based on symptomatic cases or infections. The consequent uncertainty about the case fatality risk — and hence about the severity of H1N1pdm09 — was problematic for risk assessment and risk communication during the period when many decisions about control and mitigation measures were being made.
- In addition to differences in case fatality risk estimates due to the differences in case definition (denominator), the definition of the numerator is also an important issue. Almost all of the studies in our review based the numerator on deaths among patients with laboratory-confirmed influenza infection. In contrast, most estimates of the population impact of seasonal influenza epidemics have been based on estimation of the number of excess deaths associated with influenza (i.e. estimated deaths), with the greatest annual impact in the elderly — despite influenza virus infections rarely being confirmed in this age group. The use of excess deaths rather than laboratory-confirmed deaths in the numerator of the infection fatality risk would theoretically be justified because the denominator includes all infections and not only those with a positive laboratory result. For a similar reason, deaths of patients with laboratory-confirmed infection might be a more appropriate numerator for the case fatality risk based on symptomatic case denominators.
- In preparation for the next influenza pandemic, it is essential to reach a consensus on how to define and measure the seriousness of infection (an important indicator of the severity of the pandemic), and whether the analysis can be based entirely on estimates of age-specific risk of death among cases. The consistent estimates of the infection fatality risk at around 1 to 10 deaths per 100,000 infections identified in our review may represent the seriousness of H1N1pdm09 in developed countries where data were available. Similar estimates for seasonal influenza viruses, however, are not available for comparison, and neither are estimates from less developed countries in which the seriousness profile would likely be higher.


